Chemistry of dispersants
By admin
Posted on 2011-09-21
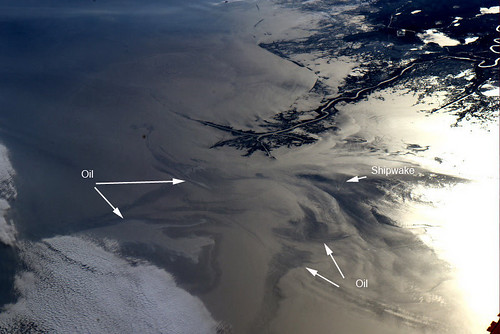
Oil Spill, Gulf of Mexico (NASA, International Space Station Science, 05/04/10)
Oil is a stew of hydrocarbon molecules. Oil doesn’t sink, it floats, and when it spills, it spreads out in a thin sheen. Parts of the oil spill, asphaltenes, froth up and emulsify in waves, becoming tarry globules of hydrocarbon chains mixed with other molecules (nitrogen, oxygen, and sulfur, as well as trace amounts of vanadium and nickel.). The spill byproducts get sticky and messy.
With 4% of the world’s population, we in the U.S. use 25% of the oil produced, spending nearly half a trillion dollars each year on oil. Neither a spike in prices, nor footage and news reports from last year’s BP oil spill changed oil usage significantly–we have cut it by just 2.4%. So, we’re not going to stop using oil any time soon, and since spills occur at every step in the oil production pipeline, they’re going to continue to occur.
So, how can chemistry help us deal with those spills?
 You may have seen Dawn commercials last summer showing workers cleaning ducks with Dawn detergent. Dawn works as a surfactant, breaking the hold the oil and tar has on the duck’s feathers, and allowing it to rinse away in water. Those are cute commercials–these oil-covered brown pelicans don’t look so cute– but how do we deal with spills on a larger scale?
You may have seen Dawn commercials last summer showing workers cleaning ducks with Dawn detergent. Dawn works as a surfactant, breaking the hold the oil and tar has on the duck’s feathers, and allowing it to rinse away in water. Those are cute commercials–these oil-covered brown pelicans don’t look so cute– but how do we deal with spills on a larger scale?
That’s what this week’s Chemistry Now videos are about. The fall release of the weekly, online, video series “Chemistry Now” is under way, and we’re uncovering dispersants as a source of interesting video and lessons. As we’ve written before, please view the video, try the lessons, and let us know what you think.
Photos: NASA Marshall Space Flight Center
Through the Chemistry Now series, NSTA and NBC Learn have teamed up with the National Science Foundation (NSF) to create lessons related to common, physical objects in our world and the changes they undergo every day. The series also looks at the lives and work of scientists on the frontiers of 21st century chemistry.
Video: On the anniversary of the final capping of the gushing oil well in the Gulf of Mexico in 2010, NBC Learn explains the chemistry of dispersants and immiscibles, in “How to Wash an Ocean.” They also mark the International Year of Chemistry with a video outlining chemistry’s “10 Big Questions,” as selected by their content partner, Scientific American.
Middle school lesson: In The Chemistry of Oil Spills, students evaluate several methods of cleanup used in the recent BP oil spill, and learn about the importance of chemistry in oil spill cleanup.
High school lesson: In the high school version of the lesson, students conduct an experiment to determine the contributing factors to the solubility of a system and the role of polarity in the solubility of a system, so that they understand the effect of dispersants on the system.
You can use the following form to e-mail us edited versions of the lesson plans:
[contact-form 2 “ChemNow]
Disclaimer: The views expressed in this blog post are those of the author(s) and do not necessarily reflect the official position of the National Science Teaching Association (NSTA).


